Purpose
The main indication of bisphosphonates (BPs) is osteoporosis treatment. However, there is growing interest in the peri- and postoperative use of BPs to mitigate total hip arthroplasty (THA) aseptic loosening (AL) risk. This systematic review aimed to evaluate the implant survival and the AL rate in patients with elective THA receiving BPs compared to those that do not receive BPs. Secondary outcomes included the comparison of revision rate, postoperative complications and patients' functional scores.
Methods
This systematic review was conducted under the PRISMA 2020 guidelines with a pre-registered PROSPERO protocol. Three engines and grey literature were searched up until May 2022. Randomized and non-randomized control trials and comparative cohort studies assessing BP and control therapy impact on THA survival were included.
Results
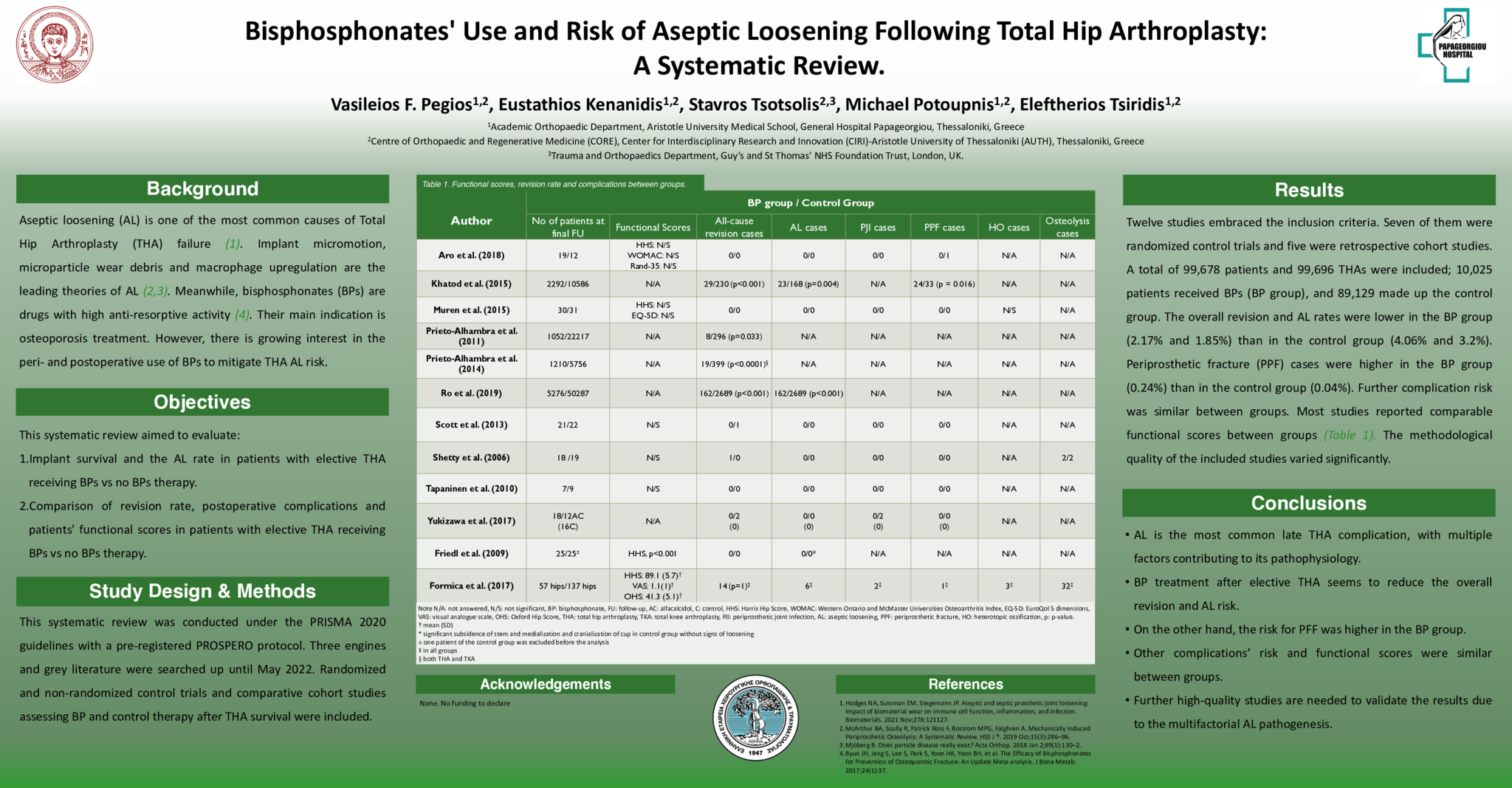
Twelve studies embraced the inclusion criteria. A total of 99,678 patients and 99,696 THAs were included; 10,025 patients received BPs (BP group), and 89,129 made up the control group. The overall revision and AL rates were lower in the BP group (2.17% and 1.85%) than in the control group (4.06% and 3.2%). Periprosthetic fracture (PPF) cases were higher in the BP group (0.24%) than in the control group (0.04%); however, the majority of PPF cases were derived from a single study. Further complication risk was similar between groups. Most studies reported comparable functional scores between groups.
Conclusion
BP treatment after elective THA seems to reduce the overall revision and AL risk. Other complications’ risk and functional scores were similar between groups. Further high-quality studies are needed to validate the results due to the multifactorial AL pathogenesis.
- 6 προβολές